The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
$ 11.99 · 4.5 (280) · In stock
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8

Vapour pressure of a solution of `5g`of non-electrolyte in `100g`water at a

Solutions (Colligative Properties, Abnormality in Molar Mass) The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol -1) in 100 g of CS, (vapour
The vapour pressure of a solution having 2.0 gram of solute X in hundred gram of CS2 is it 848.9 torr. the molecular formula of the solute is

SOLVED: The vapour pressure of a solution having 2.0 g of solute X

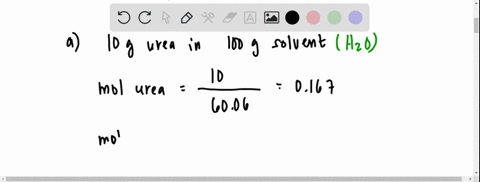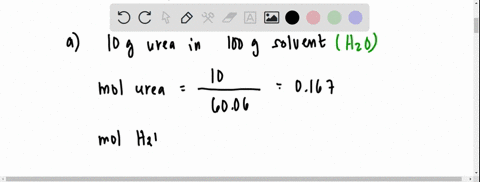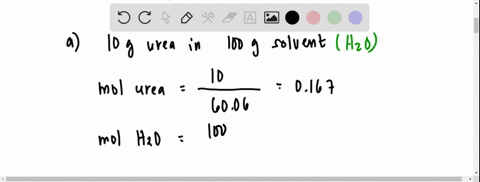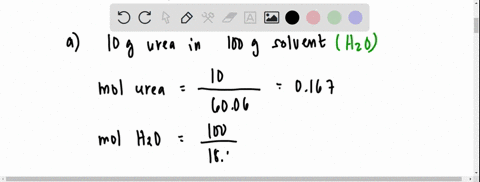
the vapour pressure of 2% aqueous solution of a non volatile substance X at 373 k is 755 torr . Calculate

The vapour pressure of CS(2) at 50^(@)C is 854 torr and a solution o
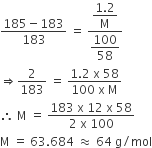
The vapour pressure of acetone at 20oC is 185 torr. When 1.2 g o
Solutions (1-47) - Final, PDF, Solubility
SOLVED: The vapour pressure of a solution having 2.0 g of solute X
Solutions (1-47) - Final, PDF, Solubility
Solved Determine the vapor pressure of a solution at
Solutions (1-47) - Final, PDF, Solubility

The vapour pressure of a solution having 2.0g of a solute X(molar mass 32gmol−1) in 100g of CS2 (vapour



