Draft Guidance Document: Applications for Medical Device Investigational Testing Authorizations
$ 12.50 · 4.5 (437) · In stock

This draft guidance document reflects Health Canada’s current thinking on Investigational Testing Authorizations (ITA) for medical devices and may be subject to changes as policy develops. The document clarifies application requirements and processes, including pre-ITA meetings, format for an ITA application and filing requests for revisions to an ITA.

An Overview Of FDA Draft Guidance On “Manufacturing Changes And Comparability For Human CGT Products

Guidance Document - Creation of the Canadian Module 1 Backbone

Ultimate Guide to UDI for Medical Devices
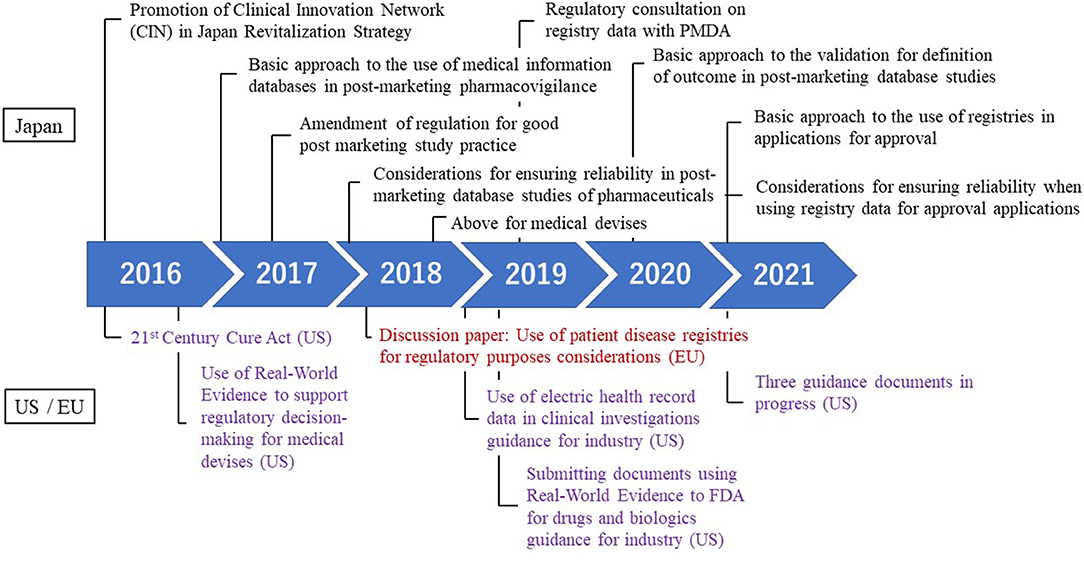
Frontiers Regulatory Approval With Real-World Data From Regulatory Science Perspective in Japan

The Lifecycle from Drug Development Through Approval Processes American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology

HIPAA Privacy Rule - Updated for 2024

HIPAA Compliance Checklist 2024: What you need to know

Clinical Trial Application and Import Requirements in India with respect to SUGAM portal.
Regulation of “Biomaterials” and Medical Devices
Overview of the Regulatory Landscape in Portugal

Canada's Health Canada - Global Regulatory Partners, Inc.

Demystifying The Investigational Device Exemption Process - Healthcare - United States

Q&A: Understanding FDA Inspections of Clinical Investigators

