At a high pressure, the compressibility factor (Z) of a real gas is us
$ 13.50 · 4.8 (254) · In stock

At high P. P gt gt (n^(2)a)/(V^(2)) So ‘a’ can be neglected.

Compressibility Chart - an overview

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Gas Compressibility Factor Calculator Excel SpreadsheetLow Cost Easy to Use Spreadsheets for Engineering Calculations Available at Engineering Excel Spreadsheets

gas laws - How to find the temperature relationship between the isotherms in a compressibility factor (Z) vs pressure graph? - Chemistry Stack Exchange

Compressibility Factor Z Important Concepts and Tips for JEE Main

Compressibility factor (z): real gases deviate from ideal behav-Turito

Gas Compressibility Factor and Control Valve Sizing
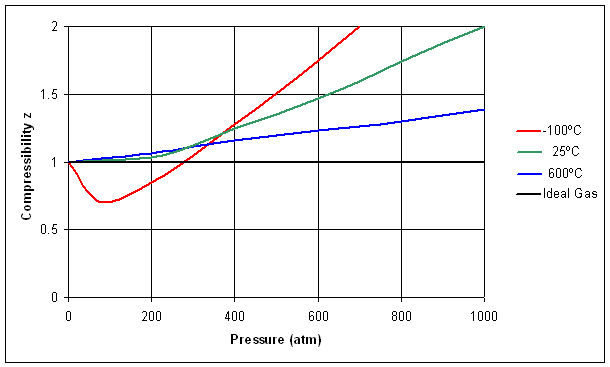
Gas Laws – First Year General Chemistry
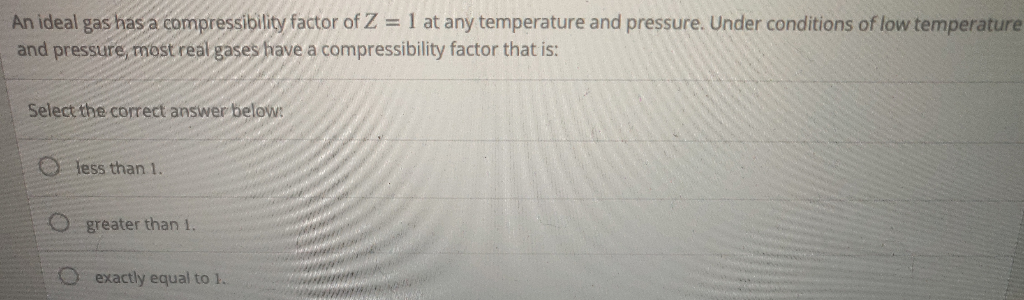
Solved An ideal gas has a compressibility factor of Z = 1 at
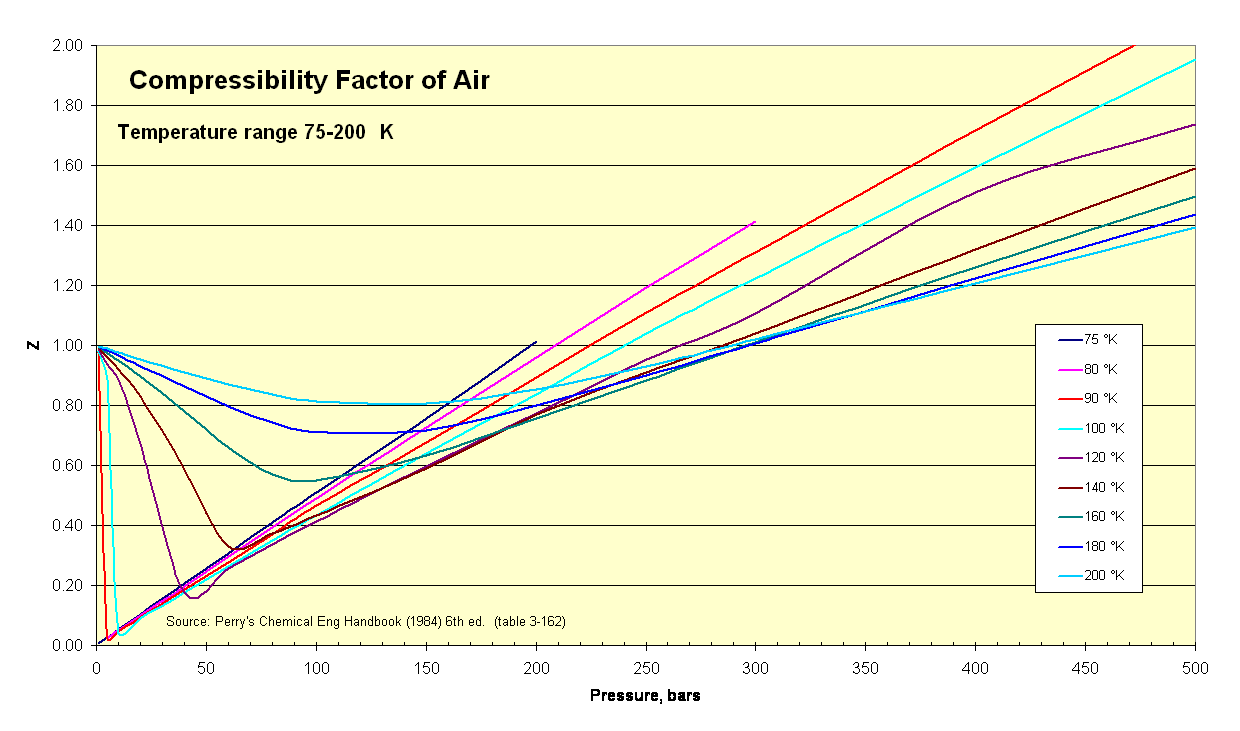
Air Compressibility Factor Table - EnggCyclopedia

Compressibility Factor - an overview

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
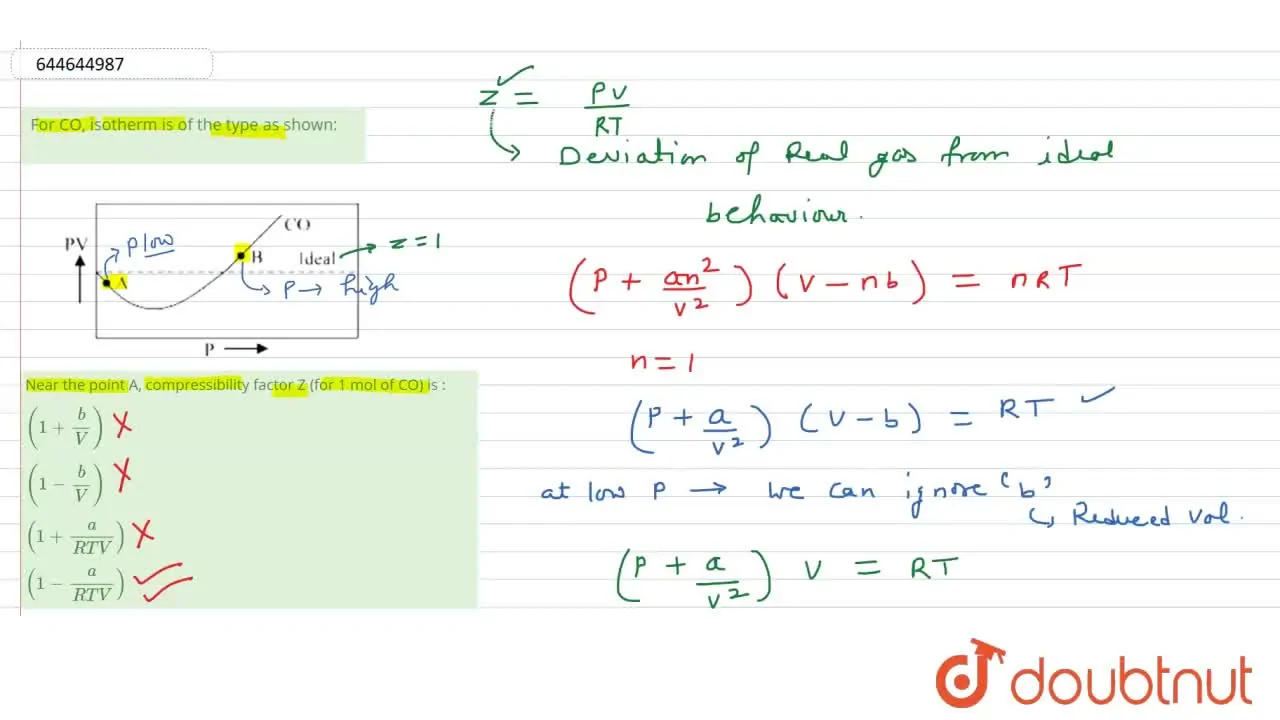
For CO, isotherm is of the type as shown: Near the point A, compr

Compressibility factor (gases) - Citizendium