24. Assertion :In B2H6, the terminal B H bonds are shorter, than
$ 24.50 · 4.5 (654) · In stock
24. Assertion :In B2H6, the terminal B H bonds are shorter, than the B H bridge bonds Reason: The terminal B H bond order is greater than that of the B H bridge bond
24- Assertion-In B2H6- the terminal B-H bonds are shorter- than the B-H bridge bonds Reason- The terminal B-H bond order is greater than that of the B-H bridge bond

A new look at the nido-undecaborate system - ScienceDirect

In which of the following compound (s) terminal (2C - 2e^{-}) bond and bridge bonds are lying in same plane

The correct statement(s) regarding diborane (B_2H_6) is/are : (a) Maximum six hydrogen atoms can

A new look at the nido-undecaborate system - ScienceDirect
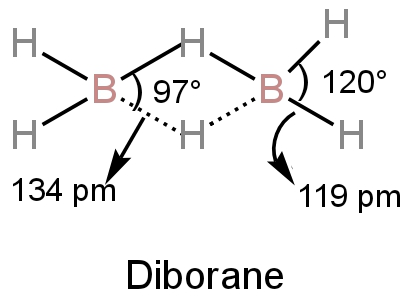
The correct statement about diborane is A All BHB angles class 12 chemistry JEE_Main

Solved] Which of the following statement is not correct about dibora
Chemical Bonding SPECIAL ASSIGNMENT, PDF, Chemical Polarity
CSIRO PUBLISHING Australian Journal of Chemistry
1 M3 2 Chemical Bonding, PDF, Ionic Bonding

