Compressibility Factor Z Important Concepts and Tips for JEE Main
$ 8.99 · 5 (477) · In stock

JEE preparation requires clarity of concepts in Compressibility Factor Z. Click here to access solved previous year questions, solved examples and important formulas based on the chapter.

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

Compressibility Factor (Z), States of Matter - L19
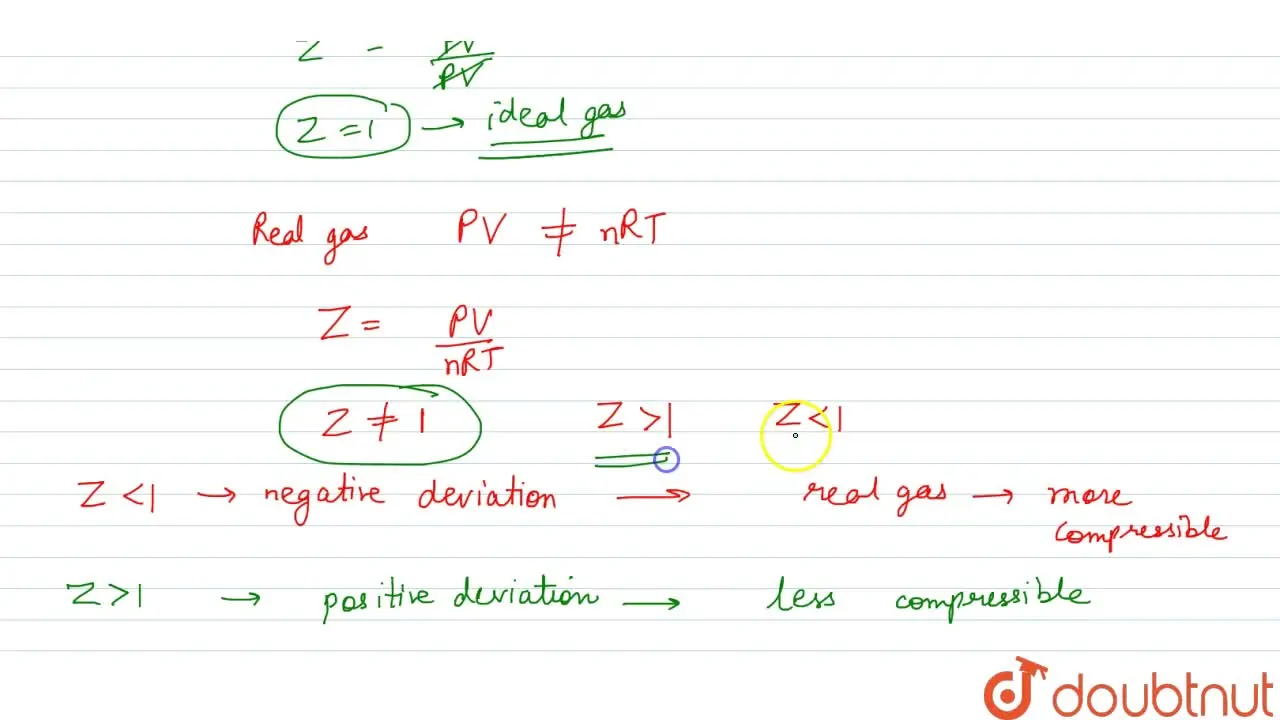
Punjabi] What is the value of compressibility factor for ideal gases
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is? 1.$\left( {1 + \dfrac{b}{V}} \right)$ 2.$\left( {1 - \dfrac{b}{V}} \right)$3.$\left( {1 + \

The compressibility factor (Z) for a gas is less than one.What does

JEE: Van der Waals Equation, Chemistry By Unacademy

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

JEE Main Chemistry Syllabus 2024 - Download Detailed Syllabus PDF