Applications for Medical Device Investigational Testing Authorizations Guidance Document
$ 30.50 · 4.7 (592) · In stock

Applications for Medical Device Investigational Testing Authorizations Guidance Document

validation and verification of medical device.pptx

Micromachines, Free Full-Text

Emerging Issues in Using Mobile Apps for Clinical Research - New York State Bar Association

Medical Device Guidelines and Regulations Handbook
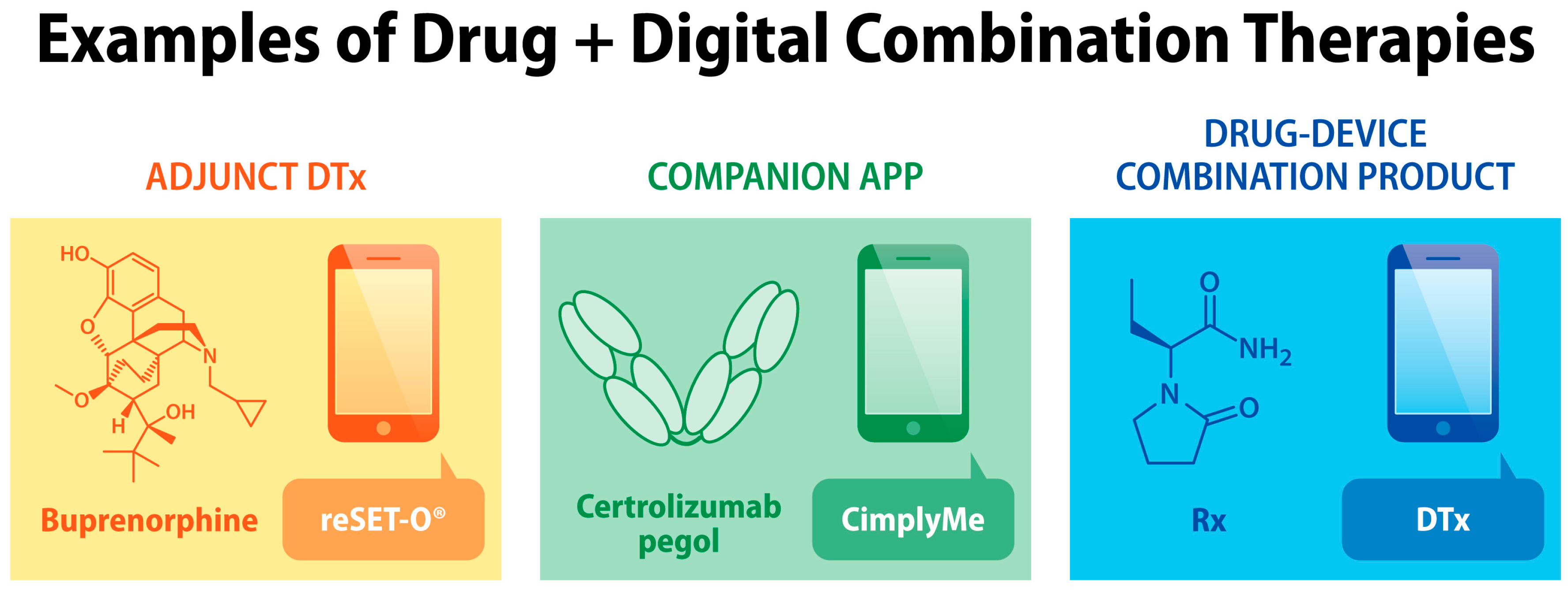
JCM, Free Full-Text
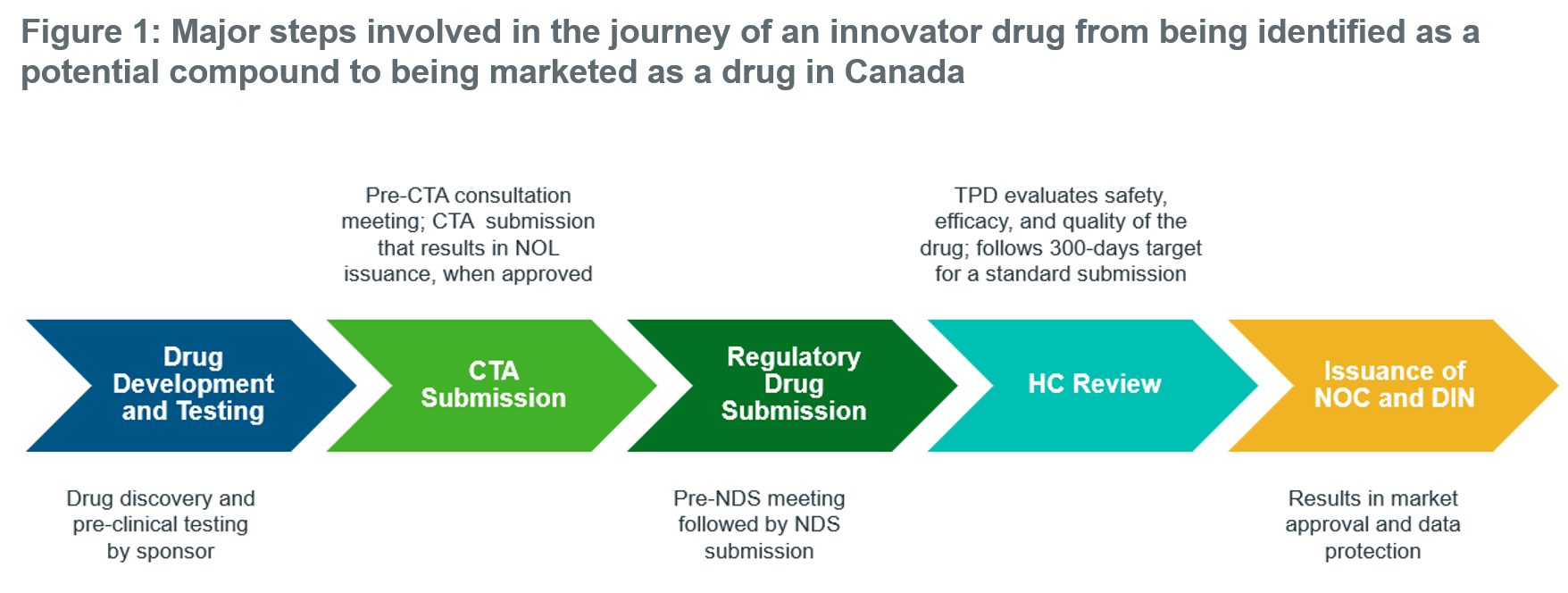
Identifying Drug Protection in Canada - IQVIA

IND Application for Botanical Drug Products - The Johns Hopkins

Class II - IV Medical Device Investigational Testing in Canada - Vantage BioTrials
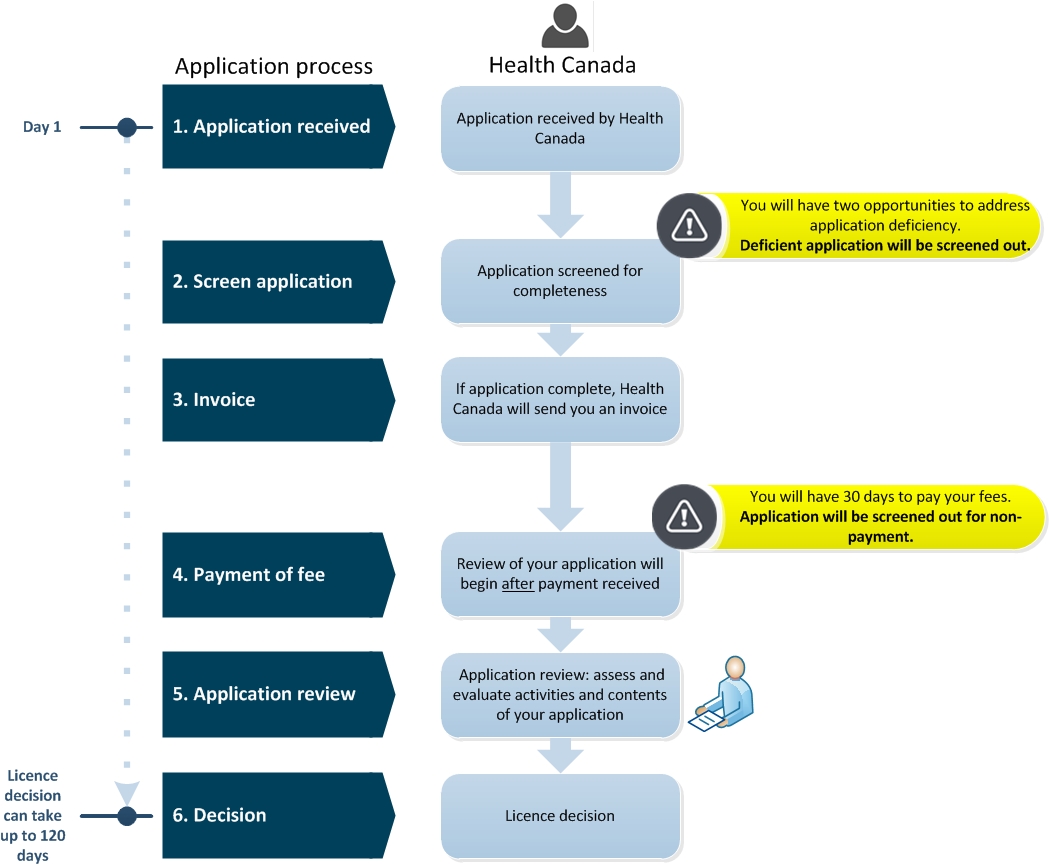
Guidance on Medical Device Establishment Licensing (GUI-0016

Health Canada Creates the Medical Device Directorate
-image.jpg)
When does My Application Qualify for an Abbreviated 510(k)?

How to get your COVID-19 Related Medical Device to Market Under FDA Emergency Use Authorization (EUA)