kinetic theory - Why doesn't Helium behave as an ideal gas
$ 25.00 · 5 (295) · In stock

I am a bit confused (might be due to some conceptual misunderstanding) as to why doesn't Helium behave as an ideal gas (it shows a deviation from the $pV$ vs $p$ graph)? (Given the fact that it is

Kinetic Molecular Theory Aim
Noble Gas - GeeksforGeeks

gas laws - Among hydrogen, helium and carbon dioxide, which gas would behave most like ideal gas and why? - Chemistry Stack Exchange
Between N2 and O2, which gas is more ideal? - Quora
Why do gases behave ideally? Is there any gas that we can say it

Why is HCl less ideal than CH4, He and N2? - Quora

Convection, Venus, Thought Experiments and Tall Rooms Full of Gas
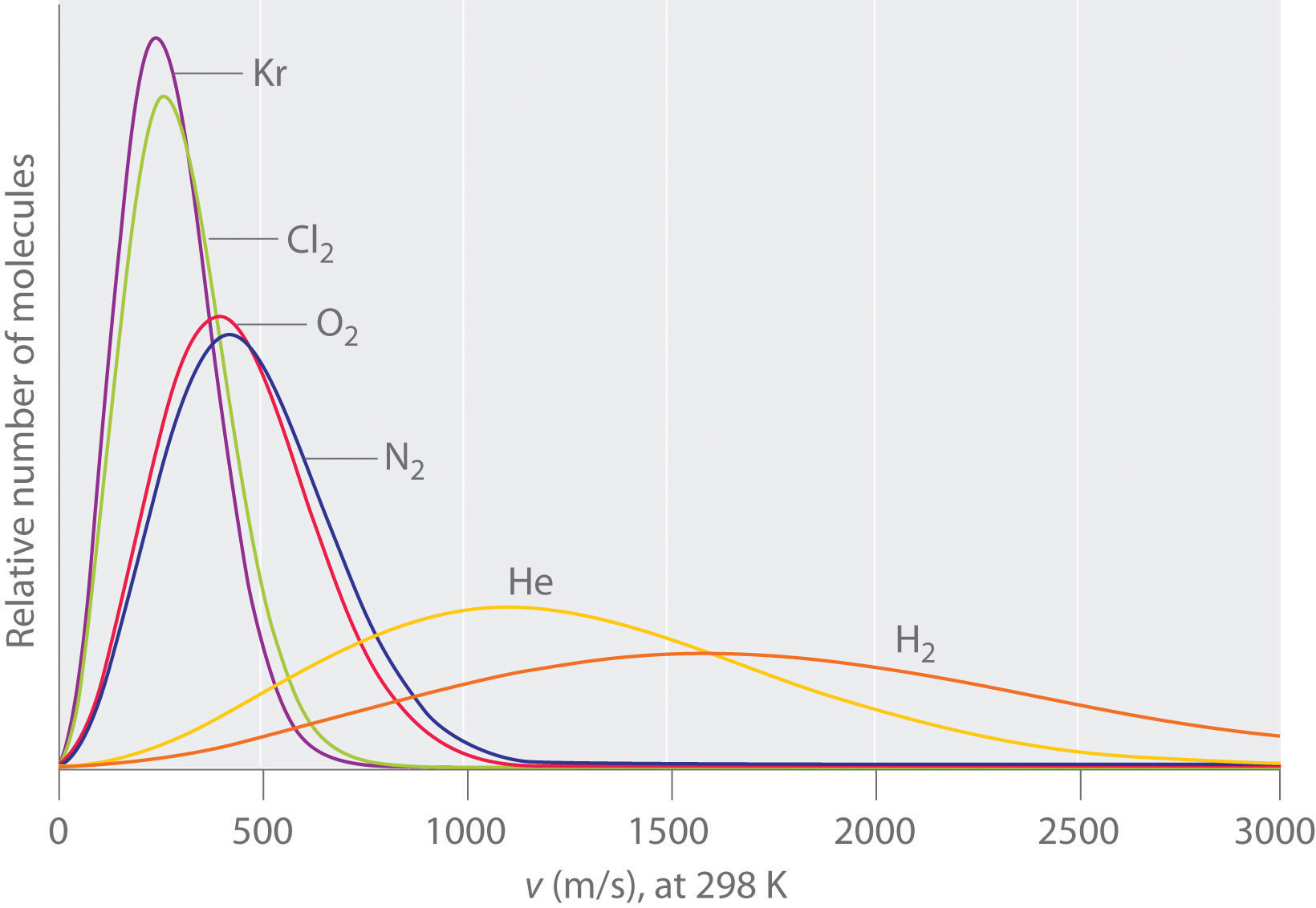
10.7: The Kinetic Theory of Gases - Chemistry LibreTexts

The Kinetic Molecular Theory - ppt video online download
Kinetic Molecular Theory - Video Tutorials & Practice Problems
Under what conditions do you expect a real gas such as hydrogen gas to behave like an ideal gas? - Quora
12.1: Introduction - Physics LibreTexts
6.3: Combining the Gas Laws: The Ideal Gas Equation and the General Gas Equation - Chemistry LibreTexts

Ideal Gas Law, Examples & Problems - Lesson
