Microbiological Media Management - SOP & Guideline - Pharma Beginners
$ 24.00 · 4.8 (329) · In stock

Standard Operating Procedure (SOP) and Guideline for the Receipt, Storage, Preparation, Growth Promotion Test, use, and Disposal of microbiological media.
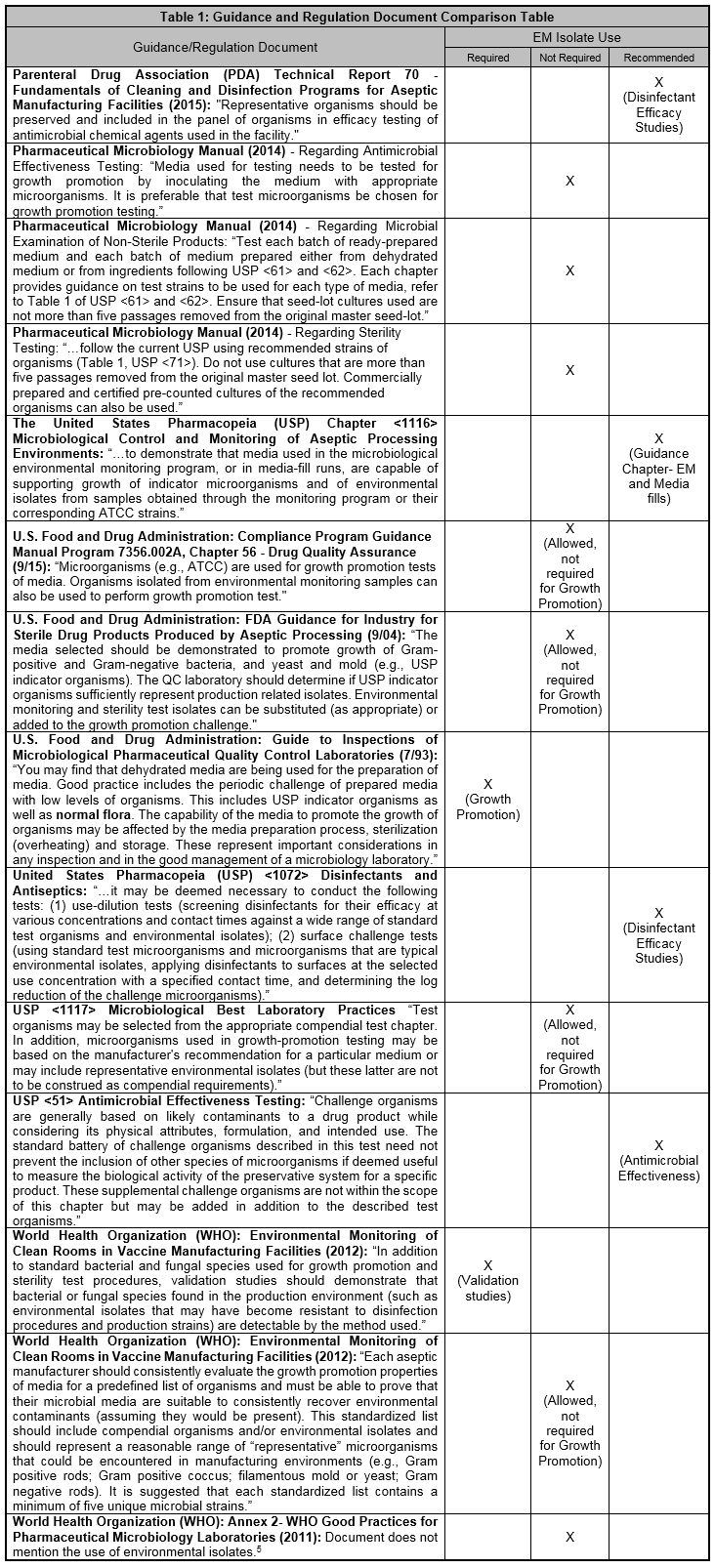
Environmental Isolates What's The Proper Use Of In-House Cultures
Sustainability, Free Full-Text

Microbiology Quality Control Testing: Definition & Procedures

Qualifying your cleanroom

SOP for Pharmaceutical Industry, Pharma SOPs

Microbiological Media Management - SOP & Guideline - Pharma Beginners
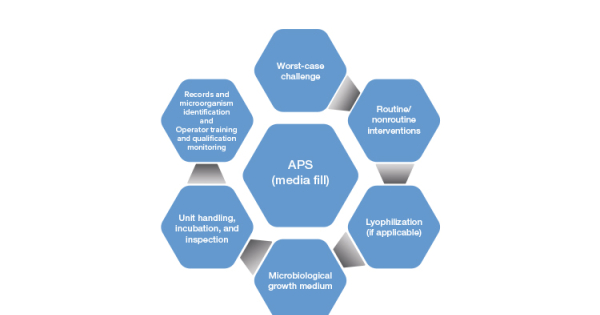
Validation of Aseptic Processes Using Media Fill
MICRO 4 SOP For Microbial Monitoring in Drain Point of Pharmaceutical Manufacturing Sites

PDF) Microbiological Culture Media: A Complete Guide for
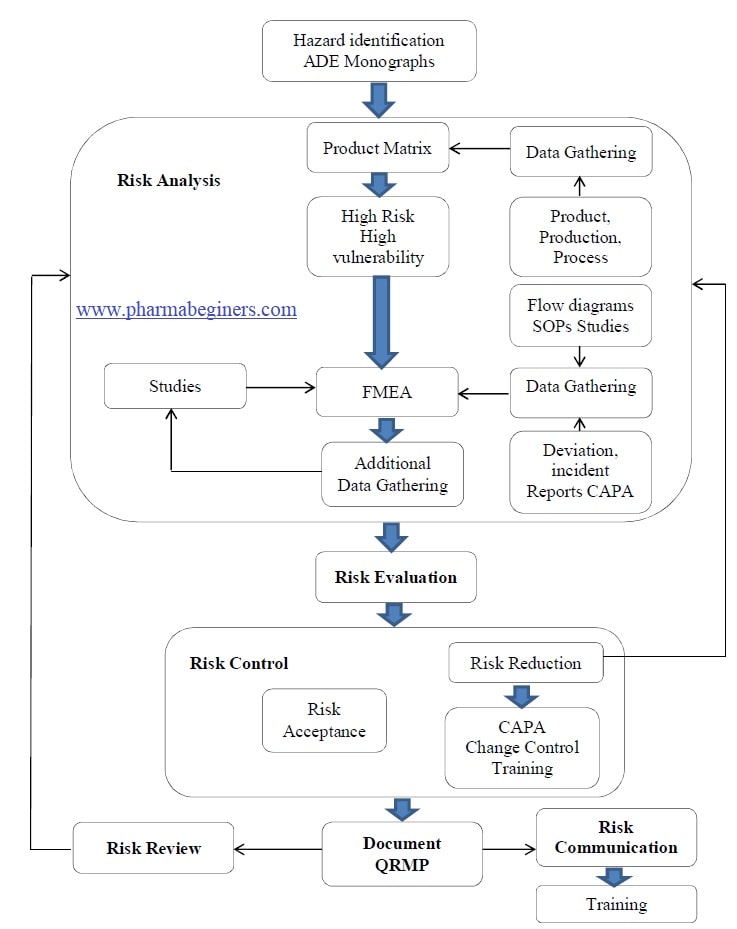
Cross Contamination, Mix-Ups & Microbial Contamination - SOP in Pharma
🏆 Mastering SOP Content: Elevating Pharma Excellence 📊💡

