Solved What is the equilibrium constant (Kp) at 45 °C for
$ 23.00 · 4.5 (268) · In stock

Answer to Solved What is the equilibrium constant (Kp) at 45 °C for
✓ Solved: Calculate ΔG^∘at 45^∘ Cfor reactions for which (a) ΔH^∘=293 kJ ; ΔS^∘=-695 J / K

The equilibrium constant `K_(c)` for the following reaction at `842^(@)`C is `7.90xx10^(-3)`. What i

Simple new correlation for the prediction of equilibrium constant (KP) of Haber reaction covering the industrial conditions - ScienceDirect
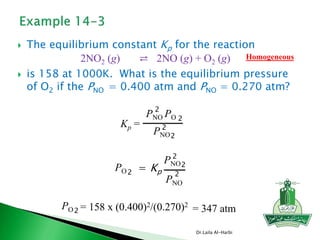
Chapter 14
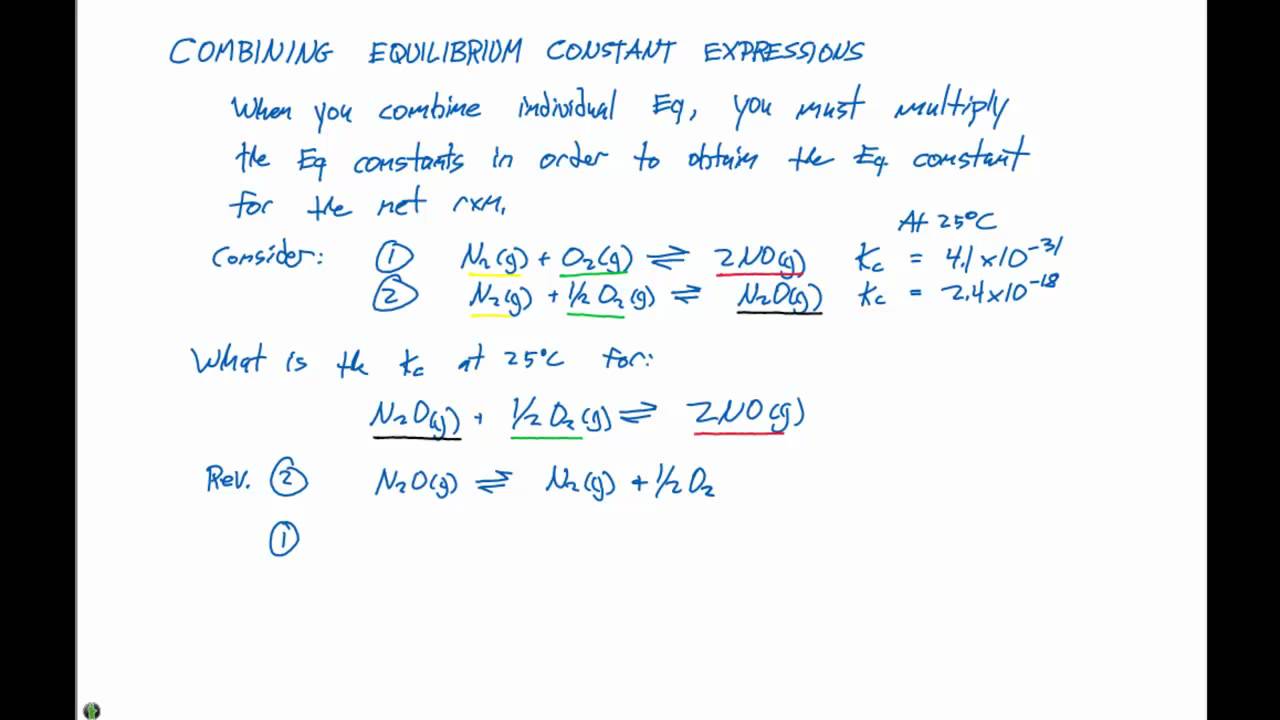
15.3 Combining Equilibrium Constants

Answered: A reaction vessel initially contains…
Solved What is the equilibrium constant, Kc, at 450°C for

Chapter 14
A well structured lesson including starter activity, AfL work tasks, main work tasks with answers on The Equilibrium Constant KpBy the end of the
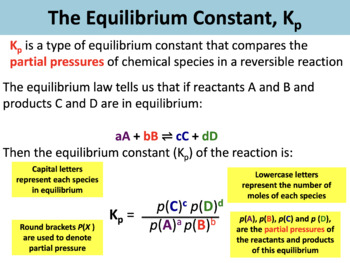
The Equilibrium Constant Kp

The equilibrium constant (K_(p)) for the reaction, PCl_(5(g))hArrPCl_(3(g))+Cl_(2(g)) is 16. If

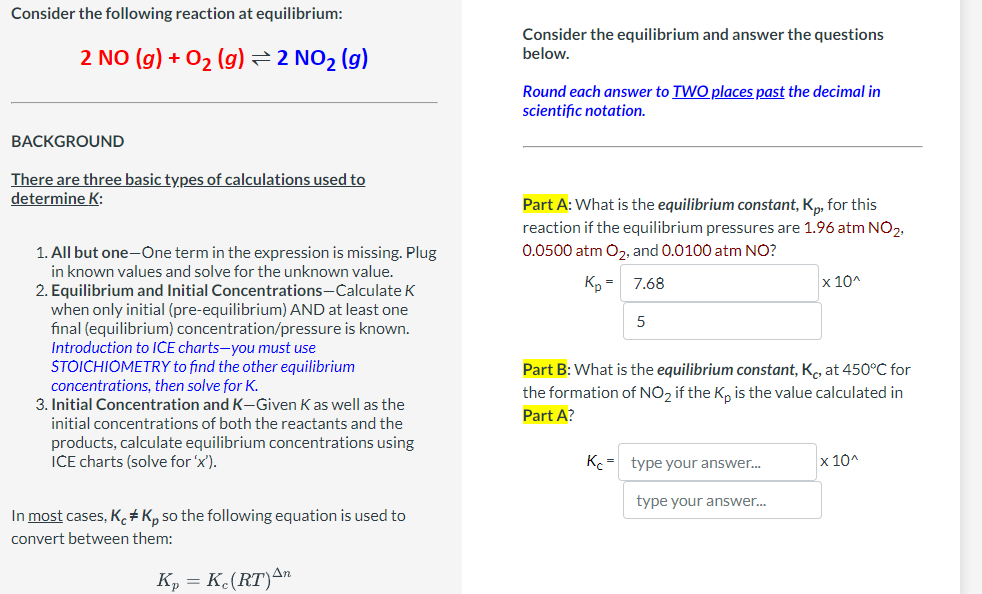
Consider the reaction: 2 NO( g) + O2( g) ∆ 2 NO2( g) The followin
At 444^° C, the equilibrium constant K for the reaction 2AB gives A2 +B2,The degree of dissociation of AB will be (A) 10

Solved 11. For the reaction the equilibrium constant, Kp -45

The equilibrium constant K(p) for the thermal dissociation of PCl(5) a