Solved 1. The compression factor, Z of a gas is 0.625. Which
$ 14.00 · 4.8 (427) · In stock

The use of GANs and transfer learning in model-order reduction of turbulent wake of an isolated high-rise building - ScienceDirect

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be

Norriseal valve sizing reference guide by RMC Process Controls & Filtration, LLC. - Issuu
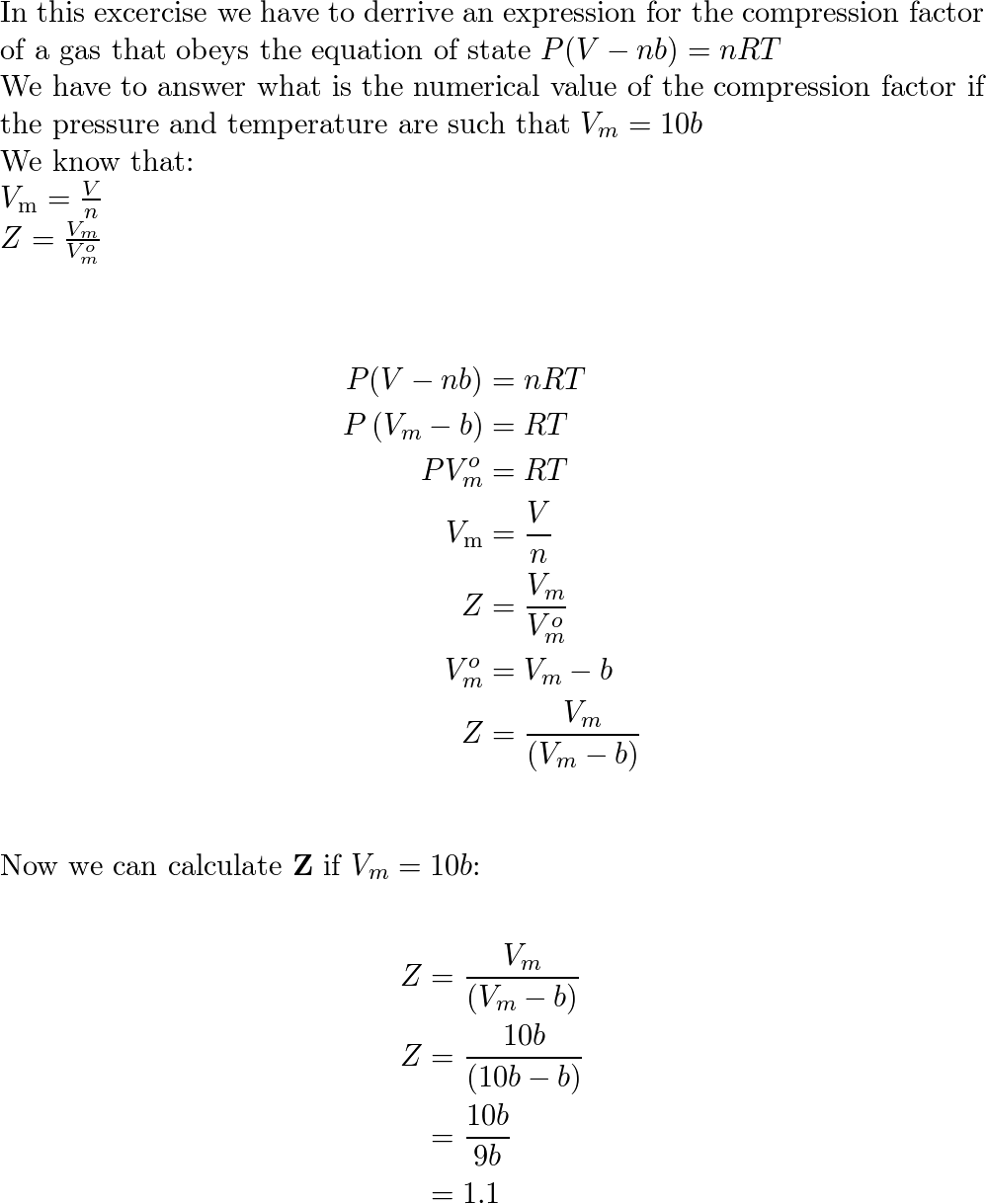
Derive an expression for the compression factor of a gas tha

The pressure of a liquid is increased isothermally. The molar volume of the liquid decreases from 50.45 andtimes; 10-6 m3/ molto 48 andtimes; 10andminus;6 m3 / mol during this process. The isothermal

gas laws - Graph of compressibility factor vs pressure when real gas is assigned Z=1 - Chemistry Stack Exchange

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Processes, Free Full-Text

Thermodynamics Fundamentals
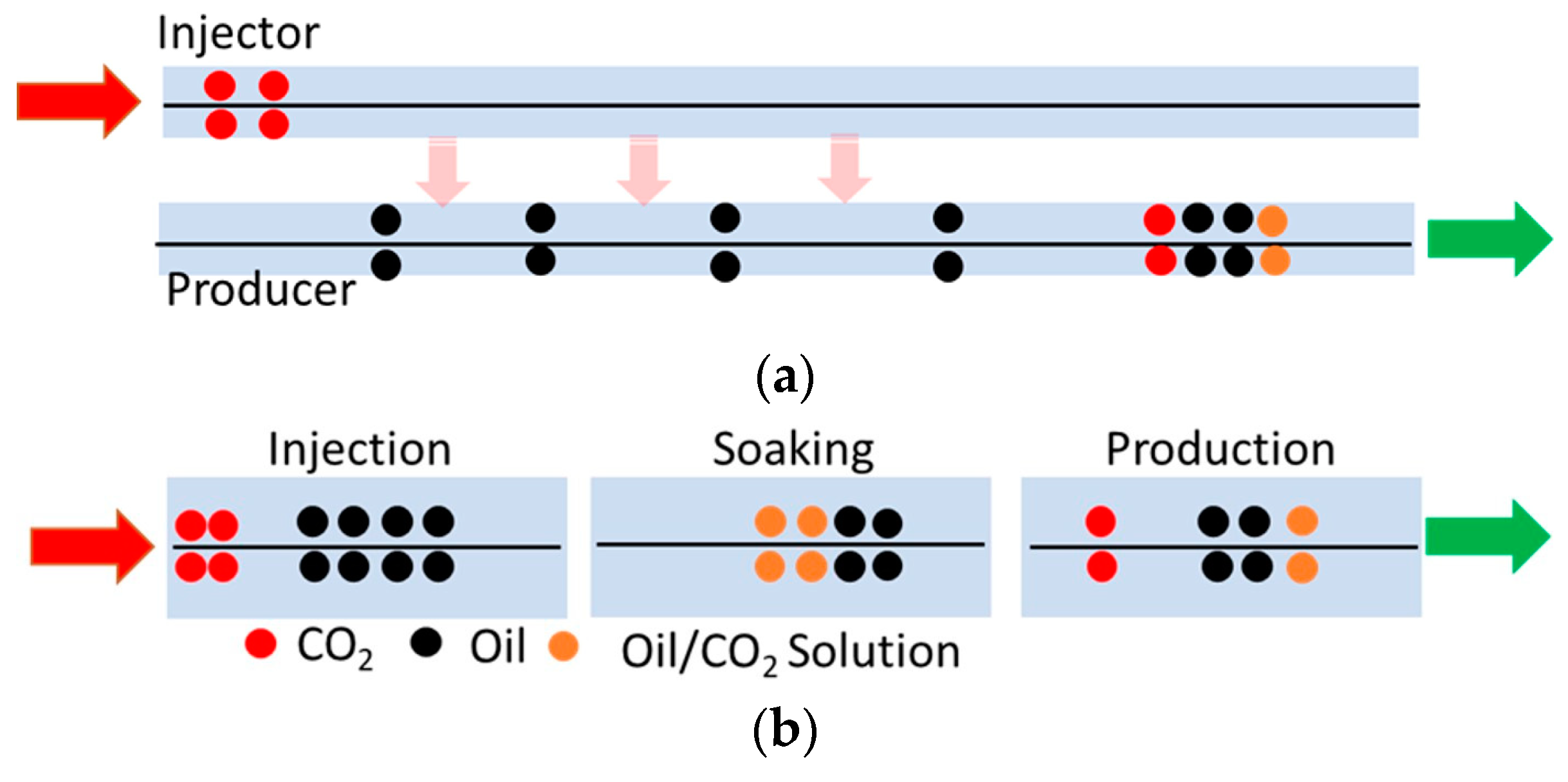
Energies, Free Full-Text
Ficoquimica, PDF, Gases

Simulation details for DMSO-[EMIM] + [DCA] − and water- [EMIM] + [DCA]

1. The compressibility factor, z, is the ratio of

Gas desorption law of granular coal in negative-pressure environment and calculation of gas loss during negative-pressure sampling - ScienceDirect
