physical chemistry - Why do some gases have lower value of Z for a
$ 14.99 · 4.7 (266) · In stock

In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m

Gas Compressibility - an overview

Replacing Plastics with Alternatives Is Worse for Greenhouse Gas
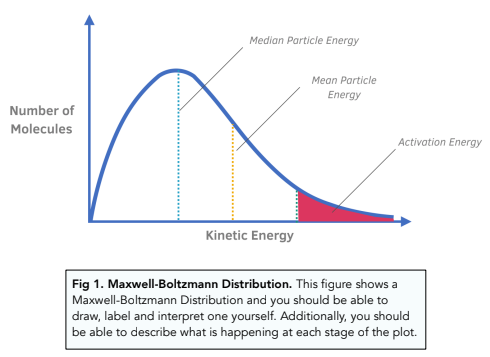
Kinetics - The Maxwell–Boltzmann Distribution and Catalysts (A-Level Chemistry) - Study Mind
If z<1, does it mean that the gases behave more like perfect or real gases? - Quora

Non-Ideal Gas Behavior Chemistry: Atoms First

Distribution of energy in the ideal gas that lacks equipartition

What is the significance of the curve part in Z vs. P graph of

Gas (Gaseous State) - Characteristics, Properties, Video, FAQs
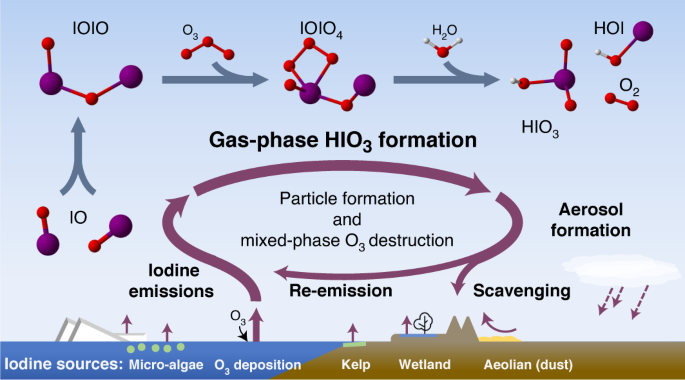
The gas-phase formation mechanism of iodic acid as an atmospheric aerosol source

Compressibility factor - Wikipedia

Compressibility factor (Z) for a van der Waals real gas at critical point is