physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
$ 24.50 · 4.9 (540) · In stock

In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m

Sustainability, Free Full-Text
822 questions with answers in PHYSICAL CHEMISTRY

Metal–organic framework - Wikipedia
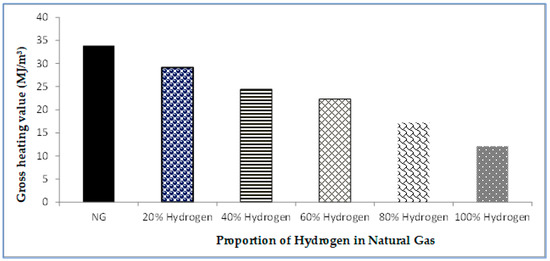
Gases, Free Full-Text
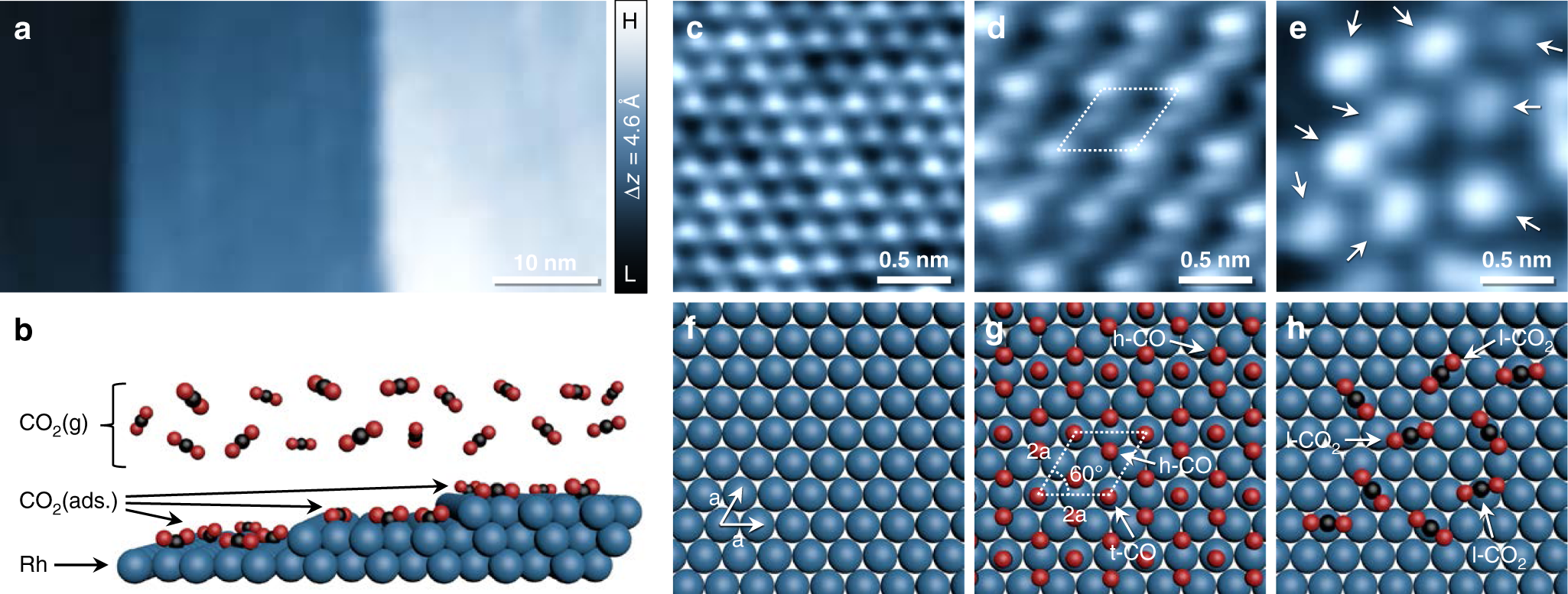
How Rh surface breaks CO2 molecules under ambient pressure

Non-Ideal Gas Behavior Chemistry: Atoms First

Multimolecular Complexes of CL-20 with Nitropyrazole Derivatives

Pressure - Wikipedia
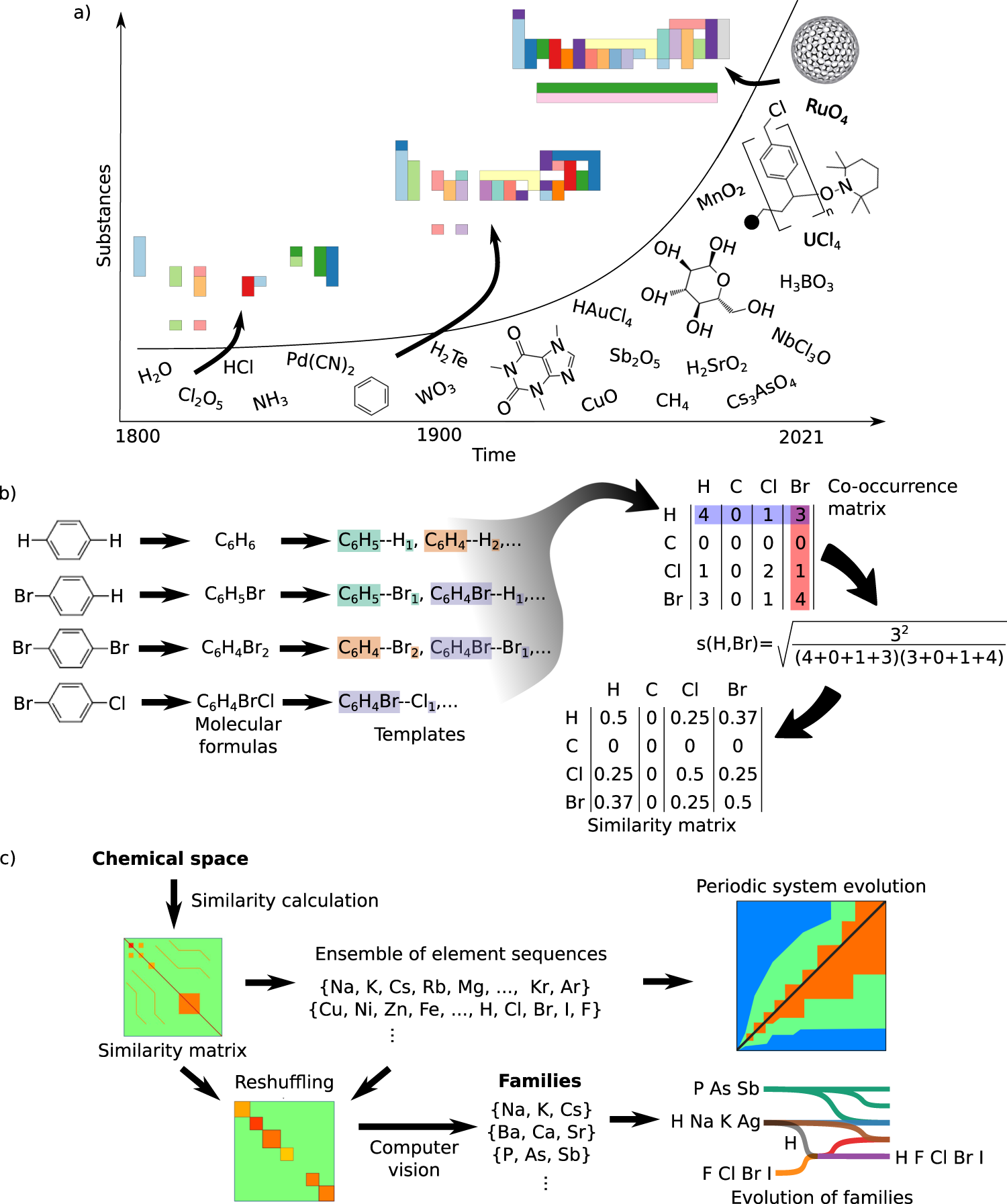
The six stages of the convergence of the periodic system to its

Activity Coefficient Definition, Equation & Examples - Lesson
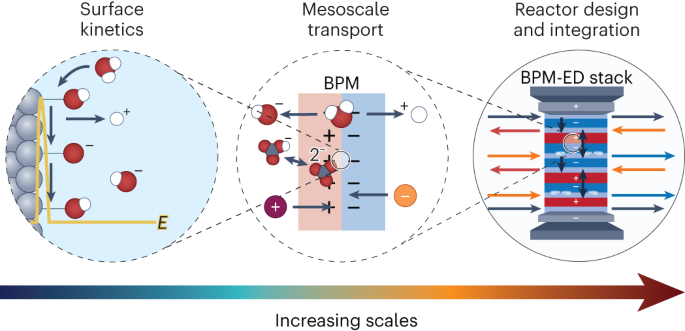
Multi-scale physics of bipolar membranes in electrochemical
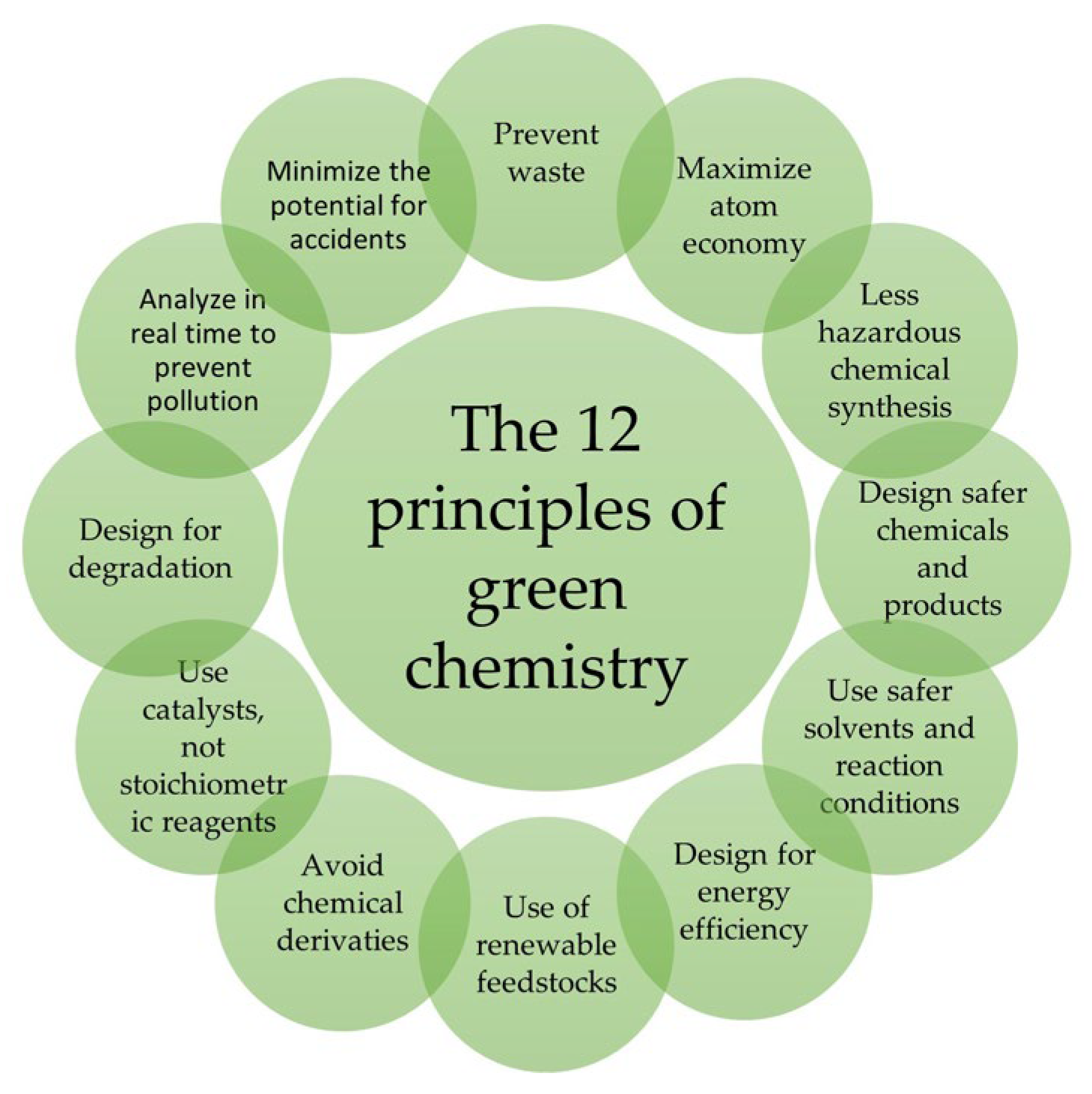
Molecules, Free Full-Text
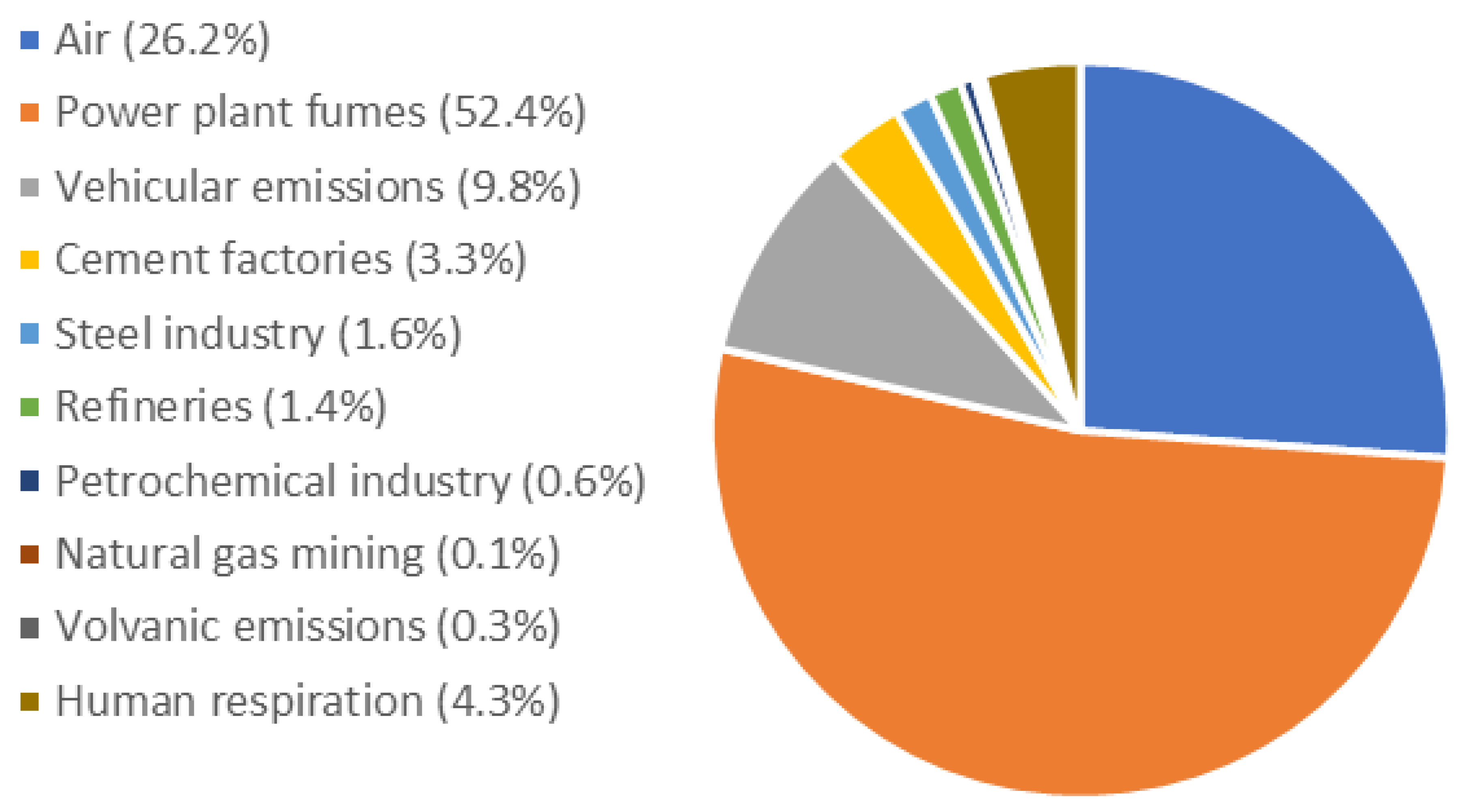
Atmosphere, Free Full-Text
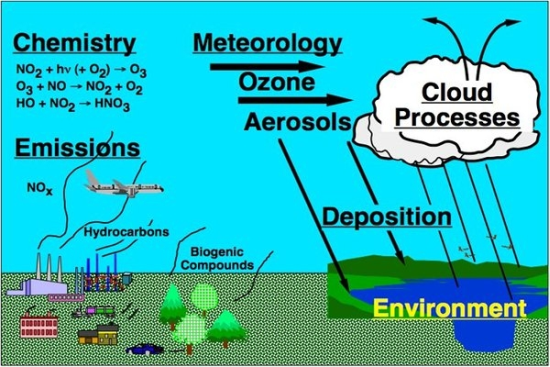
Atmosphere, Free Full-Text
