Compressibility factor (Z) for a van der Waals real gas at critical point is
$ 19.99 · 5 (98) · In stock

Share your videos with friends, family and the world

Simple Equation Real Gas Compressibility Factor Z
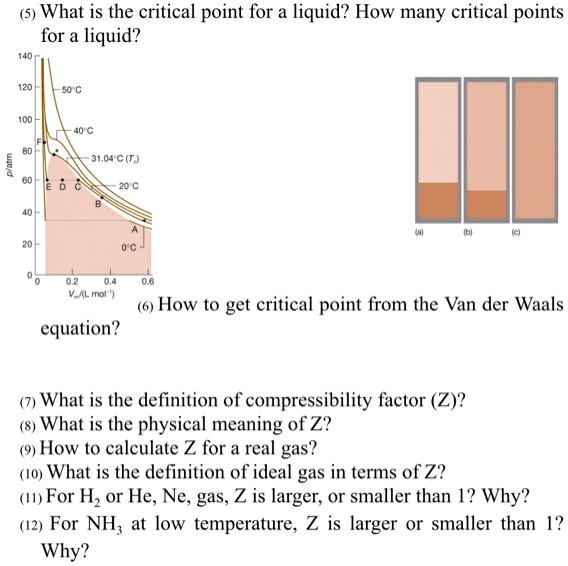
SOLVED: What is the critical point for a liquid? How many critical points for a liquid? 5 31.04 VcALmol How to get the critical point from the Van der Waals equation? (
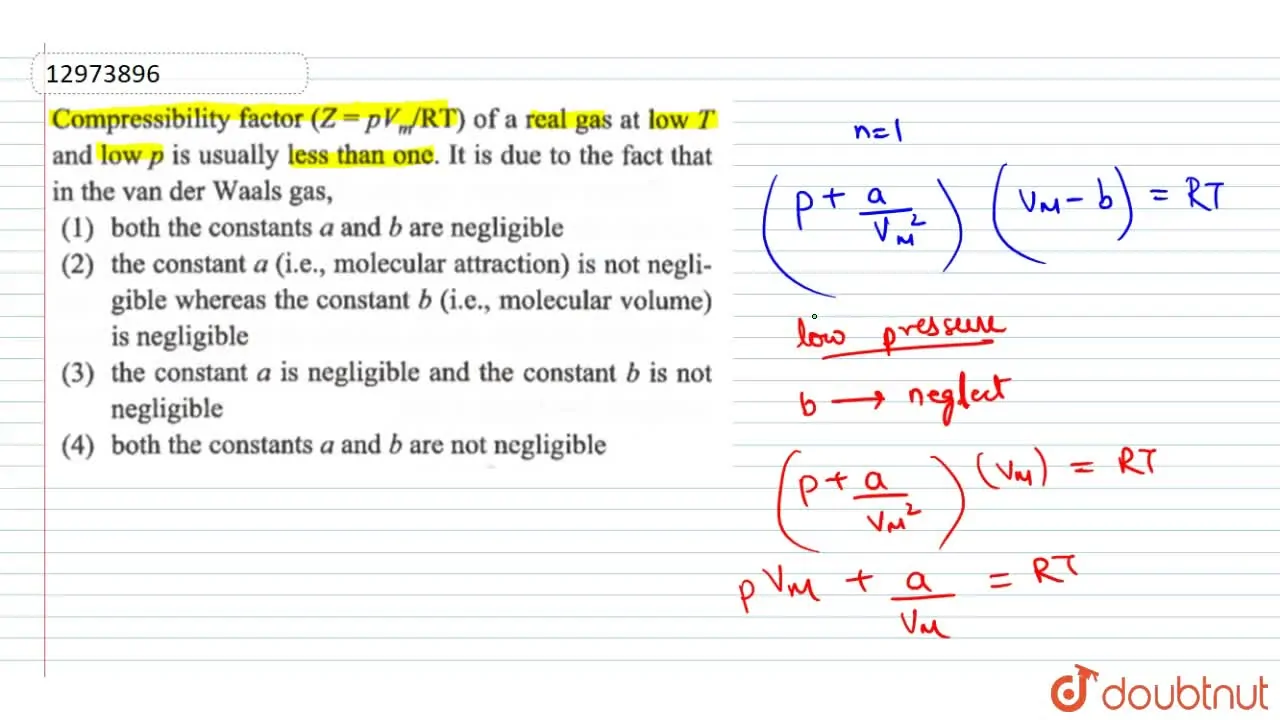
the constant a is negligible and the constant b is not negligible

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

The compressiblity factor a gas obeying van der Waals' equation of state is given by V V-b RTV (2) a ✓ RTV V-b V-b RTV (3) Va (4) RTV V-6
How can we calculate critical temperature, volume and pressure in terms of a and b? - Quora

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

Van Der Waals Equation of State - an overview
Notes - CHE 2203 Chemical Thermodynamics and Thermochemistry-1, PDF, Gases

The Compression Factor, Z, and Real Gases - What you NEED to Know!

Bengali] What will the value of compressibility factor (Z) be for a g
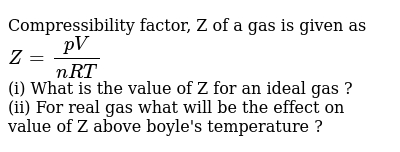
Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
Van der waals equation: Derivation, Explanation
