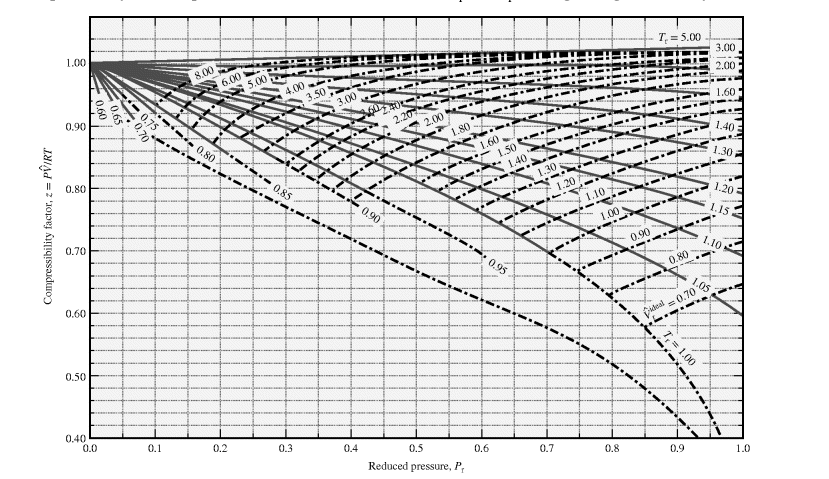
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
$ 16.50 · 5 (211) · In stock

In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state

physical chemistry - Pressure vs volume plot for real gas and ideal gas - Chemistry Stack Exchange

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
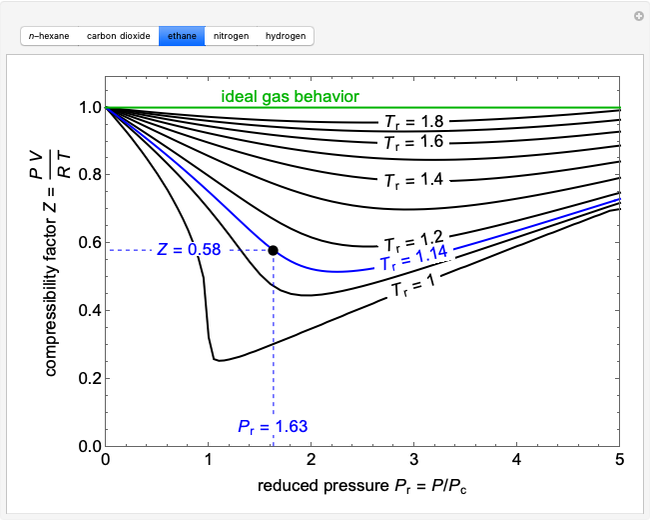
Compressibility Factor Charts - Wolfram Demonstrations Project

Aqueous Solid Formation Kinetics in High-Pressure Methane at Trace Water Concentrations

KVPY-SX 2016 Chemistry Question Paper with Solutions PDF Download
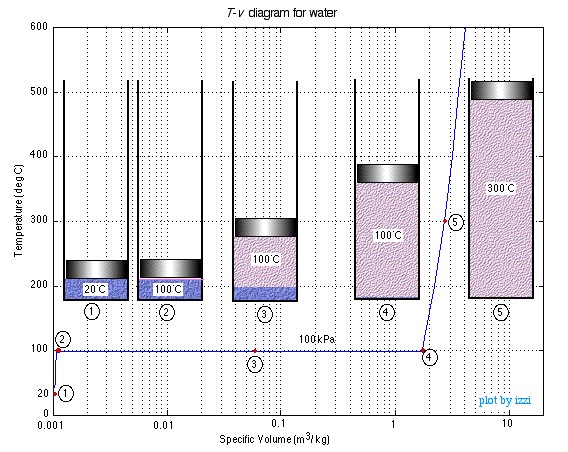
Chapter 3: Pure Substances – Thermodynamics
Solved Use the compressibility charts to answer the
Gaseous State

Compressibility factor (gases) - Knowino
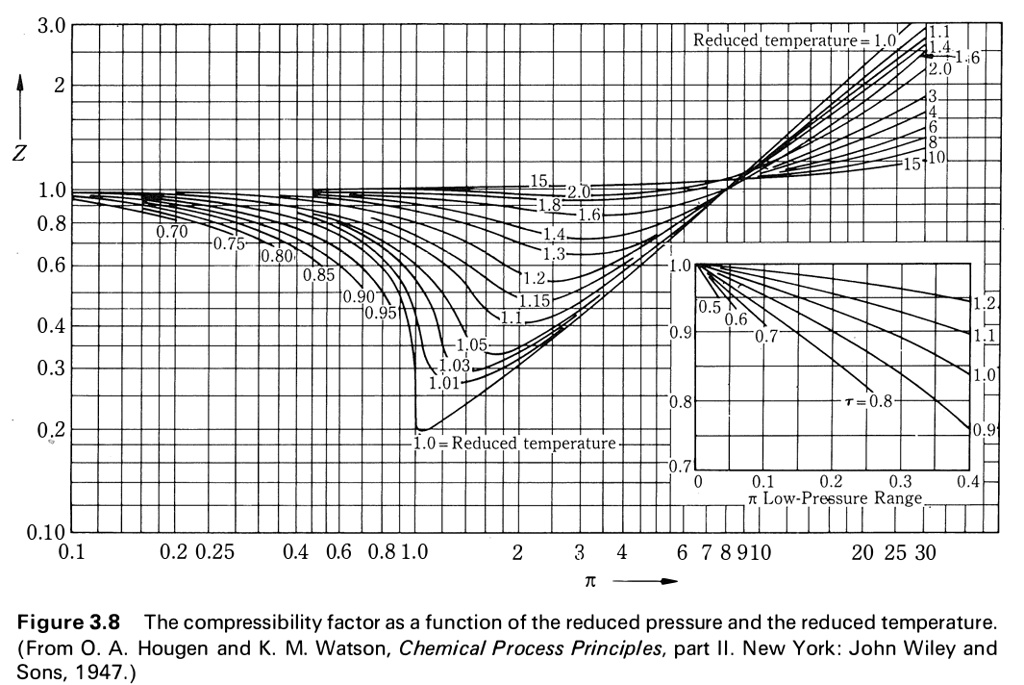
SOLVED: Use the graph of compressibility factors in terms of
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

Compressibility Chart - an overview